Hero or Villain: Unravelling the functions of Astrocytes
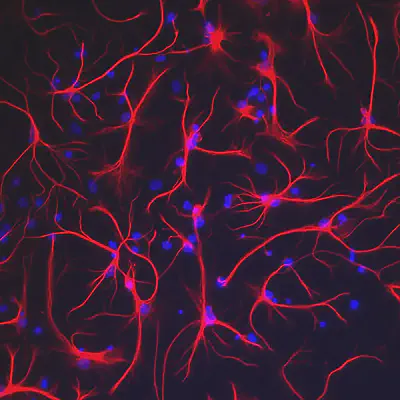
What are Glial Cells?
Glial cells are the non-neuronal, electrically inactive cells that are present in the brain. The glial cells were discovered in 1856, by German pathologist Rudolph Virchow. These cells were thought to be the supportive cells for the neurons. Initially these cells were considered to be the connecting cells in the brain and hence the name “glia” which literally translates to “glue” in Latin. It was only later that the varied functions of Glial cells were discovered. The glial cells comprise of several sub-categories of cells. These include-Astrocytes, Oligodendrocytes and the Micro Glia that are present in the Central Nervous System (CNS) and, Satellite cells and Schwann cells that are located in the Peripheral Nervous System (PNS).
Astrocytes
Astrocytes, named after their star shaped structures, are the glial cells that mediate all the neuro-vascular communications in the brain. Like most Glial cells these were also considered to be only connective tissues before their main function was discovered.
Function
Unlike the neurons which are connected to each other through ‘synapses’, the Astrocytes communicate among themselves with the help of calcium ion oscillations in gap junctions. They take part in a variety of tasks - signaling using calcium ions and transmitter molecules (cholesterol and thrombin), maintaining homeostasis of the brain and the extracellular fluid and ions in close proximity to neurons. They play an active role in ‘Immuno-Defense’ which is the building of immunity in the brain by forming reactive centers where these Astrocytes tend to encircle the damaged neurons. In several neurodegenerative diseases, astrocytes ensure that the inflammations are properly shielded from the other regions of the brain.
Neuroinflammation and Immunodefence
Astrocytes respond to a central nervous system (CNS) injury or disease via the process called ‘reactive astrogliosis’ which is a defense mechanism to minimize and repair the initial damage. This is characterized by remarkable molecular, cellular, and functional alterations in reactive astrocytes. CNS injury leads to two types of reactive Astrocytes – one type being helpful (A2 reactive Astrocytes) and other being harmful (A1 reactive Astrocytes), Hence the dual role as a “hero” and a “villain”. They are found to remain benign until they are provoked by chronic neuro-inflammation.
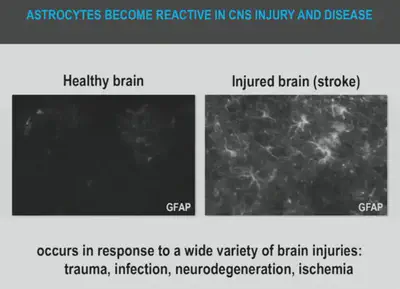
The Astrocytes become pro-inflammatory when they are exposed to certain chemicals such as LPS (Lipopolysaccharides), causing the activated microglia to release ROS (Reactive Oxygen Species), resulting in the formation of A1 Astrocytes. These A1 Astrocytes cause neuronal death. They produce high levels of inhibitory components such as neuro toxins - soluble CSPGs (Chondroitin sulphate proteoglycans), which forms chemical and physical barriers to axon elongation following CNS injuries. These CSPG deposits form a substantial barrier to regeneration and are largely responsible for the inability of the brain and the spinal cord to repair internal damages. Soluble neurotoxins rapidly kill a subset of CNS and mature Oligodendrocytes (glial cells that provide support and supplement the neurons in the CNS with myelin sheath). A1 Astrocytes are found in abundance in people affected by Alzheimer, Huntington, Parkinson and Amyotrophic related Sclerosis and Multiple Sclerosis. By inhibiting the formation of A1 astrocytes, the death of neurons can be prevented. Researchers around the globe, are working hard to unravel the multidimensional roles of reactive astrocytes as it can revolutionize the treatment of neurodegenerative diseases. Some mechanisms that are actively pursued are prevention of A1 astrocyte formation, promotion of A1 reversion and blocking the secretion of a soluble neurotoxins.
Astrocytes tend to become anti-inflammatory when they are deprived of oxygen resulting in the formation of A2 Astrocytes as in the case of strokes. These Astrocytes show an increase in the neurotrophic factors and ’thrombospondins’ at the mRNA level. Thrombospondins belong to a class of proteins that induce the neurons to form synapses, thus promoting the neurons to strengthen synapses. A2 Astrocytes tend to produce substances that support neuron health, growth and survival near the stroke site.
Neuro-Vascular Mediators
Astrocytes act as mediators between the neurons and the blood vessels, thus helping the neurons in getting important nutrients that are essential for the neuronal activities. The neurons release chemicals which are known as “neurotransmitters” such as glutamate. The Astrocytes, being receptive to the neurotransmitters, respond to them by fluctuating the calcium ion concentrations. In response to the neurotransmitters that are released by the neurons, the Astrocytes release vasodilators such as EET (Epoxyeicosatrienoic Acid), which cause the blood cells to dilate and release glucose. The released glucose is then absorbed by the Astrocyte which is sent to the neurons as lactate, where it gets converted into ATP. Almost all the muscles in the human body tend to take nutrients directly from the blood vessels by releasing vasodilators whenever they are active. On the contrary, the neurons communicate indirectly with the blood vessels with the Astrocytes acting as the mediators.
Modelling Perspective
Let’s consider a model in which the neurons are connected directly to the blood vessels without the mediating Astrocyte layer (i.e.) assuming that the neurons themselves release vasodilators. Let the model comprise of two neurons - one active and one inactive neuron and two blood vessels - one dilated and one undilated blood vessel. The brain comprises of multiple neurons that become active at varied instances of time. The activated neuron fires and the inactive ones doesn’t. The activated neurons have a greater energy demand and tend to release vasodilators which causes the blood cells to dilate and release glucose to the neurons. However, these vasodilators that are released by the neurons, tend to act on all the local blood vessels, resulting in energy release to all the neurons irrespective of whether they are active or not. This results in an imbalance between the demand and request between the neurons, where the neurons which are active don’t receive enough energy while on the contrary, the neurons that are inactive tend to receive energy. Hence, we face a supply-demand problem, when we assume one-to-one communication between the neurons and the blood vessels. The presence of the intermediate Astrocyte layer helps in solving the above problem. Astrocytes receive neurotransmitters from selective neurons and then send back lactose to those specific neurons, thereby preventing the imbalance between the demand and supply.
Through various in-vivo studies it is established that astrocytes play fundamental and multi-dimensional roles in the function and dysfunction of brain. Hence unraveling the complex and sophisticated mechanisms that govern the astrocyte functions is mandatory in not only understanding the brain operations but also their role in CNS pathology. The progress made in technology to solve the numerous differential equations (in some cases up to 20 million equations) in multiple dimensions helped to model the neuron–astrocytes interactions.
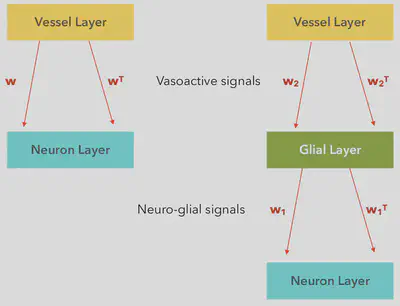
In the above model, as shown in the flowchart, the Astrocyte-neuron connections are compared to the “Deep Belief Networks” as proposed by Geofferey H. Hinton, Simon Osindero and Yee-Whey Teh (2006). The model connections are considered to be plastic (i.e.) these connections constantly evolve/change with time. Plasticity of the Astrocyte-Neuron connections and the Astrocyte-Vessel connections are considered to be of key importance. The weights which are represented by $w, w^T, w_1, w_1^T, w_2$ and $w_2^T$ are then trained by using the Hebbian principle of learning. According to this principle, the Neuronal and Neuro-Vascular communications are considered to strengthen as the the number of interactions between them increase. Once the weight are optimized, then the algorithm can be used on real life examples.
Conclusion
Previously, all Glial cells were assumed to comprise more than 90% of the whole brain, which leads to an average of 8-10 glial cells for a given neuron.However recent studies studies have shown that there might be an 1:1 ratio between the number of neurons and Astrocytes. As the neurons and the Astrocytes are connected, we need the Input patterns for the neurons and the Feedback patterns to the neurons to be similar. Over the years, the understanding about the Astrocytes has evolved from being considered as mere neuronal glue, to being acknowledged for their role in Neuro-vascular communication and to recently being considered for their important role in neural computation. Thus, the perceptions about these Astrocytes have come a long way. Neuroscientists around the world continue to remain fascinated and enthralled at the extent of the Astrocyte’s significance in the information processing of our brain. The depths of its influence in the neuronal computation continues to awe Scientists till date. Researches on Neuro-astrocyte communication has now become one of the most important and sought-after regions of research in the domain of Neuroscience. Indeed, it is going to be an interesting quest!
This article was originally published in Synkranti.
References
- Progressive fibrinoid degeneration of fibrillary astrocytes associated with mental retardation in a hydrocephalic infant. W S Alexander. Brain 72(3):373-81 (1949)
- CEquations for InsP3 receptor-mediated [Ca2+] oscillations derived from a detailed kinetic model: a Hodgkin-Huxley like formalism. Yue-Xian Li and John Rinzel (1993)
- Shaping inhibition: activity dependent structural plasticity of GABAergic synapses. Carmen E. Flores and Pablo Méndez (2014)
- Neurotoxic reactive Astrocytes are introduced by activated microglia. Nature 541, 481-487 (2017)
- Elusive role for reactive Astrocytes in neuro degenerative diseases. Lucile Ben Haim, Maria-Angeles Carrillo-de Sauvage, Kelly Ceyzériat, Carol Escartin (2015)
- Genomic Analysis of reactive Astrogliosis. Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA (2012)
- Neural Computation. Gandrakota, Chakravarthy, Pradhan (2010)
- Vascular Dynamics Aid a Coupled Neurovascular Network Learn Sparse Independent Features: A Computational Model. Frontiers in Neural Circuits.Philips RT, Chhabria K, Chakravarthy VS. (2016)